| Author |
|
Wayde Murray
Byrne Robotics Member

Joined: 14 October 2005
Location: Canada
Posts: 3115
|
| Posted: 22 March 2011 at 10:09am | IP Logged | 1
|
|
|
That diagram wouldn't allow for electron sharing, though. If electrons can be shared between atoms to create and build molecules, then the outermost shells of the adjacent atoms must be connected by whatever pattern the electrons themselves follow. Rather than two discrete spheres, perhaps they would be have to be more like two teardrops connected at the thin ends. If those dumbell-like constructions had enough energy that they could tumble over each other, the substance would be a liquid. Held from tumbling, it would be a solid.
|
| Back to Top |
profile
| search
|
| |
Michael Todd
Byrne Robotics Member

Joined: 07 September 2009
Location: United States
Posts: 4114
|
| Posted: 22 March 2011 at 10:10am | IP Logged | 2
|
|
|
Sometimes a cigar is just a cigar and teleportation is just teleportation. You cannot over think what is essentially make believe.
|
| Back to Top |
profile
| search
| www
|
| |
Wayde Murray
Byrne Robotics Member

Joined: 14 October 2005
Location: Canada
Posts: 3115
|
| Posted: 22 March 2011 at 10:17am | IP Logged | 3
|
|
|
From another article, this one discusses the different bonds. Covalent bonds are described - In covalent bonding, electrons are shared between the molecules, to saturate the valency. The simplest example is the H2 molecule, where the electrons spend more time in between the nuclei than outside, thus producing bonding. So if the two pointed-teardrops image is correct, the shared electron(s) would spend most of their time within the teardrop points as opposed to circling one atom or the other of the pair. Once again, this "bridge" indicates a physical connection, and I don't see how the bridge could be substantially longer than the atomic radii themselves. The bridge gives the pairing enormous strength, so it can't itself be flimsy. edit fur spellun
Edited by Wayde Murray on 22 March 2011 at 11:37am
|
| Back to Top |
profile
| search
|
| |
John Byrne

Grumpy Old Guy
Joined: 11 May 2005
Posts: 135485
|
| Posted: 22 March 2011 at 11:58am | IP Logged | 4
|
|
|
That diagram wouldn't allow for electron sharing, though.•• The diagram precisely represents the description you posted. Important to remember, too, that the familiar model of the atom is just that, a model. Something that gives scientists a way of describing the actions of the various parts -- which are, themselves, only models. We have no idea what an atom really "looks like".
|
| Back to Top |
profile
| search
|
| |
Michael Roberts
Byrne Robotics Member

Joined: 20 April 2004
Location: United States
Posts: 14893
|
| Posted: 22 March 2011 at 12:19pm | IP Logged | 5
|
|
|
Yikes! No!!The radius of a circle is half its diameter. If the radius of an atom is half the distance between adjacent atoms, then that distance would be equal to the diameter of the atoms. ------ That section of the page isn't written clearly, but the atomic radius is half the distance between the nuclei of adjacent, identical atoms.
|
| Back to Top |
profile
| search
|
| |
John Byrne

Grumpy Old Guy
Joined: 11 May 2005
Posts: 135485
|
| Posted: 22 March 2011 at 12:23pm | IP Logged | 6
|
|
|
Then they would be touching --- so why have physicists made so much fuss all these years about the atoms in collapsed stars and material like neutronium being so close they are actually touching, if that is the normal state of a solid?
|
| Back to Top |
profile
| search
|
| |
Michael Roberts
Byrne Robotics Member

Joined: 20 April 2004
Location: United States
Posts: 14893
|
| Posted: 22 March 2011 at 12:53pm | IP Logged | 7
|
|
|
Then they would be touching --- so why have physicists made so much fuss all these years about the atoms in collapsed stars and material like neutronium being so close they are actually touching, if that is the normal state of a solid? ---- I think they are referring to the idea that in those states, with just neutrons, the actual nuclei of the atoms are touching.
|
| Back to Top |
profile
| search
|
| |
Wayde Murray
Byrne Robotics Member

Joined: 14 October 2005
Location: Canada
Posts: 3115
|
| Posted: 22 March 2011 at 12:54pm | IP Logged | 8
|
|
|
Collapsed (degenerate) matter has the electrons forced inward until they are touching the nucleus, joining with the protons and forming nothing but a sea of neutrons. Hence, neutronium. All the empty space between the nucleus and electron shells is gone, so a given amount of matter takes up a much, much smaller volume. Staying with your earlier analogy, JB, the empty football field is now totally covered in houseflies! Pressures at Jupiter's core force the atoms of hydrogen gas together until they shed their electrons, creating metallic hydrogen. Temperatures are too low for fusion to occur, so Jupiter is sometimes refered to as a protostar. LINK. There's lots of interesting stuff that happens at a high enough gravity. edit to add link
Edited by Wayde Murray on 22 March 2011 at 1:03pm
|
| Back to Top |
profile
| search
|
| |
Jon Stafford
Byrne Robotics Member

Joined: 29 December 2010
Posts: 843
|
| Posted: 22 March 2011 at 1:02pm | IP Logged | 9
|
|
|
Solid objects have their molecules locked in place, but not their atoms. the constituent parts of atoms are always in motion relative to one another. And atoms are, as JB says, mostly empty space.
The transporters on Star Trek, if I remember correctly, work on the idea that matter and energy are in fact two states of the same thing. So matter can be transmuted into energy, "beamed" to a specific location, and then re-transmuted back into matter again. If Nightcrawler's teleportation (my spell checker doesn't like that word either) had been described as working in that manner then the "materializing in a solid object" problem would seem to apply. As OHOTMU describes it, it does not.
To answer your question about traversing distances, JB, I think OHOTMU said that the pocket dimension through which Kurt travels is much smaller than our own, so he doesn't have to "travel" a comparable distance through it. And time may not operate the same way there, explaining why his teleports seem instantaneous to us.
I was thinking maybe the having-to-see-where-he's-going limitation was entirely psychological. Kurt likely doesn't know the exact manner in which his power works, so it would stand to reason that teleporting into a solid object would seem like a real danger.
But none of this explains why he felt safe 'porting around the mansion and never worried about "landing" inside someone!
Edited by Jon Stafford on 22 March 2011 at 1:05pm
|
| Back to Top |
profile
| search
|
| |
Wayde Murray
Byrne Robotics Member

Joined: 14 October 2005
Location: Canada
Posts: 3115
|
| Posted: 22 March 2011 at 1:07pm | IP Logged | 10
|
|
|
Jon, some solids are nothing but atoms. Carbon crystals (diamonds) are made of carbon atoms and nothing else. Each carbon atom holds onto 4 other carbon atoms through covalent bonds. Imagine a a stool with 3 short legs and a post the same length as the legs sticking up out of the seat. Attach other stools at each post or leg, and continue building. Nothing but atoms and covalent bonds, and the hardest naturally occuring substance we have on earth. Coal and graphite are also pure carbon with different crystal patterns from diamond. Refined metals like gold and silver are just gold atoms and silver atoms. They are in molecular form (ore) prior to refining, atomic form (pure metal) after refining. There are lots of examples of "atoms only" all around you. (10K gold, or 14K or 18K are molecules, but pure 24K gold is just gold)
Edited by Wayde Murray on 22 March 2011 at 1:13pm
|
| Back to Top |
profile
| search
|
| |
Wayde Murray
Byrne Robotics Member

Joined: 14 October 2005
Location: Canada
Posts: 3115
|
| Posted: 22 March 2011 at 1:16pm | IP Logged | 11
|
|
|
Another bit on metallic hydrogen that includes a definition of "metal" that describes iron atoms as being "in contact" with each other. LINK
|
| Back to Top |
profile
| search
|
| |
Jon Stafford
Byrne Robotics Member

Joined: 29 December 2010
Posts: 843
|
| Posted: 22 March 2011 at 4:29pm | IP Logged | 12
|
|
|
Wayde, obviously we don't know each other, but I have spent a LOT of time being educated in the sciences! I am way more familiar with concepts like "covalent bonds" than I care to be! Frankly it just takes up space in my brain that could be used for other things, like where I left my keys! Anyway...
Atoms are mostly empty space. And even "pure" substances such as gold (the carat weight is irrelevant, by the way) aren't just atoms sitting around. They still form molecules, sometimes quite complex ones. A molecule of gold looks like this, for example:
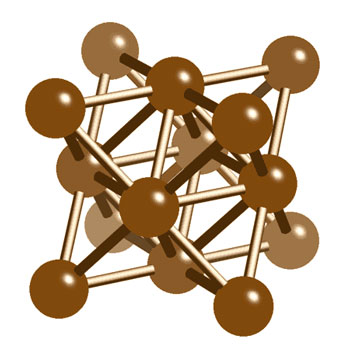
And a graphite molecule looks like this:
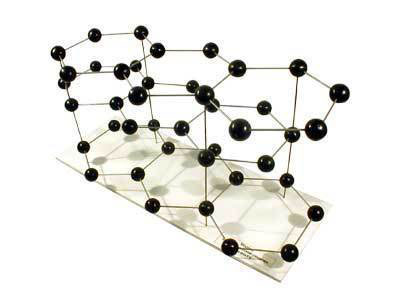
How a molecule behaves is determined by its structure and shape. Diamond and graphite are made of the same atoms, for example, but their molecules are very different, and therefore the substances behave differently. Just try giving your wife a graphite ring and you'll see what I mean!
The interaction between these shapes is what we call chemical reactions. In organic molecules, such as proteins, changing the shape -- and therefore function -- of a molecule, such as a protein, is called denaturing it. You denature protein every time you fry an egg, changing the albumin from a clear liquid (well, more or less) to a white solid. But I'm getting off track.
The point is that there is a tremendous amount of empty space in what we call "solid matter." In fact it is mostly empty space.
But still none of this really explains Nightcrawler's fear of teleporting into a solid object!
|
| Back to Top |
profile
| search
|
| |





